
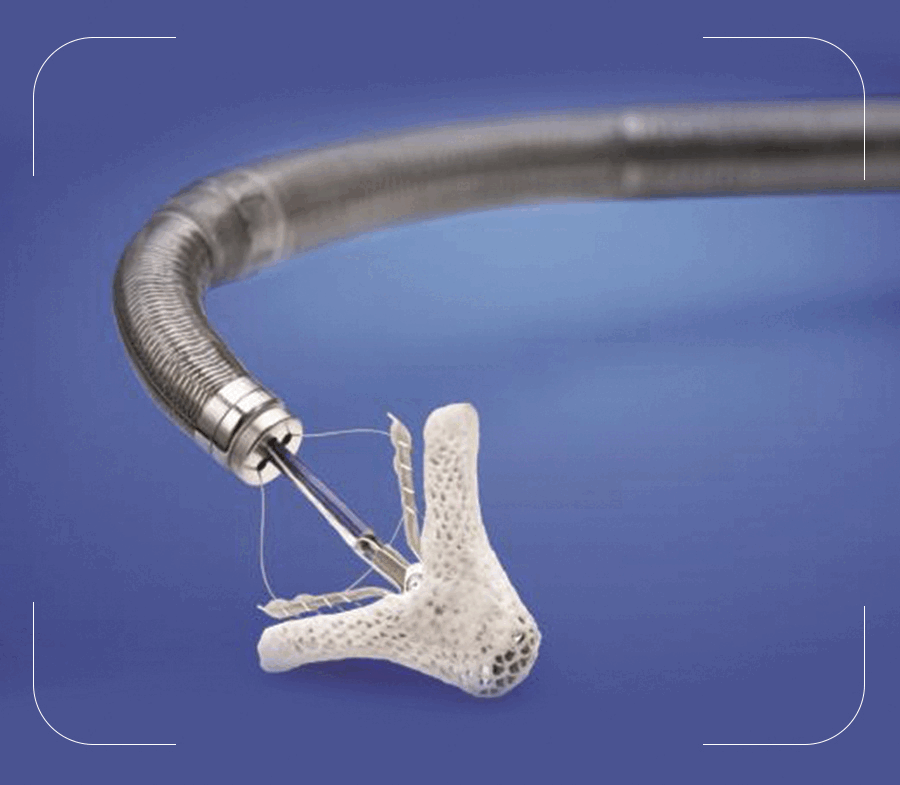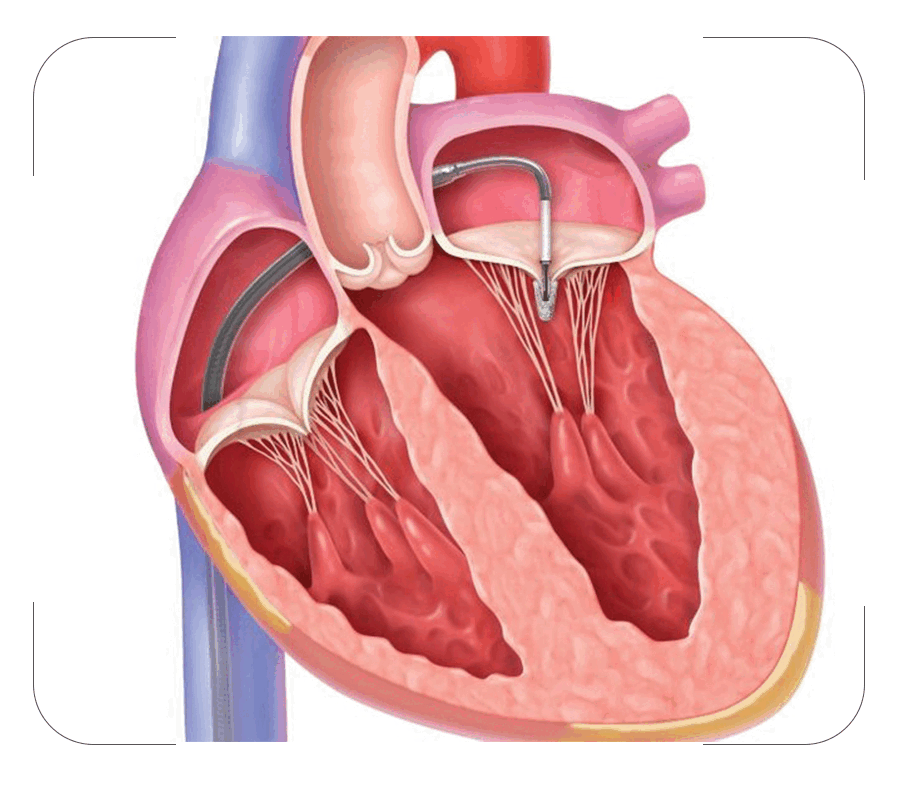
Mitral valve regurgitation (valve leaking) is a common heart disease in clinical practice. In this condition the valve between the left heart chambers doesn't close completely and it allows blood to leak backwards which results in breathing difficulty and heart failure. Common causes for mitral valve regurgitation are Rheumatic heart disease, mitral valve prolapse, Coronary artery disease and infections in the heart valve etc.
severity of mitral regurgitation can be confirmed with an echocardiogram. Conventional treatment option for this disease was open heart surgery in which the damaged valve is replaced with a metalic or tissue valve. But surgery is considered to be high risk for patients with advanced age and other co morbidities. Introduction of Mitra clip is a great hope for many thousands of elderly patients with severe valve leak and heart failure who are unable to undergo open heart surgery.
Mitra clip repairs the damaged mitral valve without opening the chest or heart. In this procedure a small metal clip is attached to the mitral valve through a pin hole via a vein in the leg. The clip stays there permanently and helps the valve to function properly again.
Previously this treatment was available only in developed countries and it is introduced to india very recently. Metromed international cardiac centre (MICC) has done the first successful mitra clipping procedure in Kerala. MICC is always there in the front line in introducing advanced treatments for various heart diseases in Kerala.
FAQ
Leaky valve (technically called mitral regurgitation) is a common condition that affects one of the valves in your heart most commonly—the mitral valve. About 1 in 10 people aged 75 and older have moderate or severe mitral regurgitation.1 To determine if you have mitral regurgitation and to assess the function and condition of your heart and mitral valve, your cardiologist may perform diagnostic evaluations, including:
- Taking a chest x-ray and/or ultrasound to see the size and shape of your heart and evaluate your lungs
- Evaluating you for symptoms of congestive heart failure (such as shortness of breath or fatigue) or other related heart conditions
You will be evaluated by a specially trained heart team, including an interventional cardiologist and a clinical cardiologist, who will review your medical history and perform a variety of tests. There are several factors they will take into consideration when deciding whether or not you are eligible for open-heart surgery and therefore a possible candidate for MitraClip™ therapy, such as your age, frailty, and the condition of your heart. Your doctor may decide that the MitraClip™ procedure is not appropriate for you if you:
- Cannot tolerate medications that thin the blood or prevent blood clots from forming
- Have an active infection or inflammation of the mitral valve
- Have mitral valve disease as a result of rheumatic fever
- Have a blood clot in your heart or in the vessels that carry blood from the lower body to the heart
Your doctor will discuss with you whether any of these issues will prevent you from having the MitraClip™ procedure. An evaluation of your heart will also confirm whether your heart valve anatomy allows for successful placement of the device.
The MitraClip™ device is a small metal clip covered with a polyester fabric that is implanted on your mitral valve. The clip is inserted through a catheter, without the need to temporarily stop your heart. There can be up to 4 different clip sizes used to tailor your clip size to your valve.
More than 150,000 patients have been treated with the MitraClip™ device worldwide.* More than 30,000 of these patients have been followed through multiple studies and registries.†
The results are impressive:
SAVES LIVES: 33% reduction in relative risk of mortality.1
FEWER HOSPITALIZATIONS: 51% relative risk reduction in heart failure hospitalization.1
HELPS PATIENTS FEEL BETTER: 2.5x more likely to experience a large improvement in quality of life.2
And it’s proven to be safe:
MINIMALLY INVASIVE: This procedure does not require opening the chest or temporarily stopping the heart.
QUICK PROCEDURE: The implantation procedure typically lasts 1 to 3 hours.4
SHORT HOSPITAL STAY: Patients are usually released from the hospital within 1 to 3 days, significantly less time compared to surgery.4
Patients who were studied 5 years after the MitraClip™ procedure continued to experience improvement in their heart function, quality of life, and ability to perform day-to-day tasks.
In the days before your procedure, it is important that you:
- Take all of your prescribed medications
- Tell your doctor if you are taking any other medications
- Make sure your doctor knows of any allergies you have
- Follow all instructions given to you by your doctor or nurse
Clinical data from patients who underwent the MitraClip™ procedure demonstrate an immediate reduction of mitral regurgitation. You should experience a significant improvement in your leaky valve–related symptoms and quality of life soon after your procedure. It is important to discuss what to expect following the procedure with your transcatheter mitral valve repair (TMVr) heart team.
No, you will not be able to feel the implant.
It is possible that you may be able to have aa magnetic resonance imaging (MRI) scan performed. However, you must inform your MRI facility and technician that you have a MitraClip™ device. After the procedure you will be given an implant card that describes what types of MRIs you will be able to receive. If the test can be performed, they may need to make certain adjustments to ensure your safety.
In a clinical study called COAPT, heart failure patients with mitral regurgitation who were treated with MitraClip™ and medication experienced improvements over those treated with medication alone.
The COAPT trial demonstrated that MitraClip™:
- Saves lives: 33% relative risk reduction in mortality
- Reduces hospitalizations for heart failure: 51% relative risk reduction in heart failure hospitalization
- Can help you feel better: 2.5x more likely to experience a large improvement in quality of life
- Is safe and well tolerated: 96.6% freedom from device-related complications at 12 months

